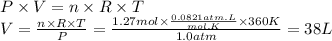Answer:
38 L
Step-by-step explanation:
There is some info missing. I think this is the original question.
Consider the chemical reaction: C(s) + H₂ O(g) ⟶ CO(g) + H₂ (g). How many liters of hydrogen gas is formed from the complete reaction of 15.2 g C? Assume that the hydrogen gas is collected at a pressure of 1.0 atm and a temperature of 360 K.
Step 1: Write the balanced equation
C(s) + H₂ O(g) ⟶ CO(g) + H₂ (g)
Step 2: Calculate the moles corresponding to 15.2 g of C
The molar mass of C is 12.01 g/mol.
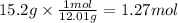
Step 3: Calculate the moles of H₂ produced from 1.27 moles of C
The molar ratio of H₂ to C is 1:1. The moles of H₂ produced are 1/1 × 1.27 mol = 1.27 mol.
Step 4: Calculate the volume of H₂
We will use the ideal gas equation.