Answer:
B 6.15g.
Step-by-step explanation:
As per the chemical equation, the reaction ratio between O2 and CO^2 is 2:1, which indicates there is 1 mole of CO^2 produced for every 2 moles of O2 responded.
Now we have to apply the molar mass and mass of O^2 to determine the moles of O^2 i.e
As we know that
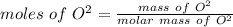
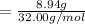
= 0.2794 mole.
So, the moles of CO2 that produced is
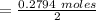
= 0.1397 mole
Now we have to apply the moles and molar mass CO^2 to find out the mass of CO^2 which is shown below:
As we know that
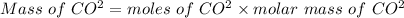
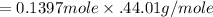
= 6.15 g