Answer:
The total surface area of these N spherical droplets is 4.4929 m²
Step-by-step explanation:
From the information given :
assuming that :
30 cm³ of gasoline is atomized into N spherical droplets &
each with a radius of 2.0 × 10−5 m
We are tasked to determine the total surface area of these N spherical droplets
We all known that:
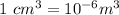
Therefore
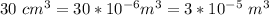
For each droplet; there is a required volume which is =
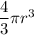 since it assumes a sphere shape .
since it assumes a sphere shape .
Thus;
replacing radius(r) with 2.0 × 10−5 m; we have:
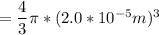
=
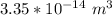
However; there are
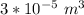 gasoline atomized into N spherical droplets with each with radius 2.0 × 10−5 m
gasoline atomized into N spherical droplets with each with radius 2.0 × 10−5 m
For N ; we have ;
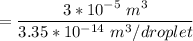
=
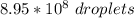
So; each droplet have a surface area =
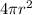
=
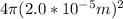
=
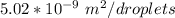
The surface area per droplet is equivalent to
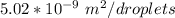
Thus;
The total surface area of these N spherical droplets will be :
=
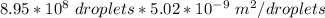
= 4.4929 m²
The total surface area of these N spherical droplets is 4.4929 m²