Answer:
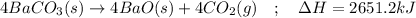
Step-by-step explanation:
Hello,
In this case, in order to answer to the requirement, we first should invert the given reaction since it is the formation of barium carbonate and we need its decomposition:
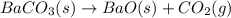
Thereby, the enthalpy of reaction is inverted, to positive since it is the contrary reaction:
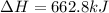
Nevertheless, we need to specify it for the formation of 4 moles of carbon dioxide it means:
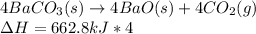
Which finally results in the following thermochemical expression:
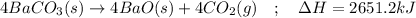
Regards.