Answer:
c. 0.033T - 2.6 °C
Step-by-step explanation:
Hello,
In this case, we should conclude that the energy lost by the water is gained by the ice to get melted, thus, we can write them in terms of melting and cooling enthalpies:
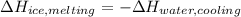
First term includes melting enthalpy of ice that is 333.89 J/g and the second one specific heat of water that is 4.18 J/g°C, therefore, we obtain:
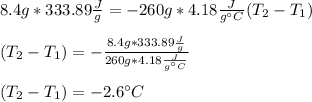
Thus, answer should be c. 0.033T - 2.6 °C since it includes the temperature decrease of water due to the undergoing cooling.
Best regards.