Answer:
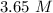
Step-by-step explanation:
We have to remember the molarity equation:
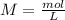
So, we have to calculate "mol" and "L". The total volume is 100 mL. So, we can do the conversion:
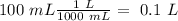
Now we can calculate the moles. For this we have to calculate the molar mass:
O: 16 g/mol
H: 1 g/mol
C: 12 g/mol
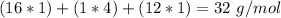
With the molar mass value we can calculate the number of moles:
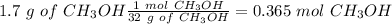
Finally, we can calculate the molarity:
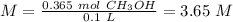
I hope it helps!