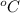Answer:
327
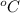
Step-by-step explanation:
The temperature at which the volume would be doubled is 327
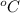 .
.
Using the formula from Charles' law:
V1/T1 = V2/T2, where V1 = initial volume, T1 = initial temperature, V2 = final volume, and T2 = final temperature.
In this case, V2 = 2V1, T1 = 300 K (27 + 273), and T2 is what we are looking for.
Substituting the different entities in the equation:
V1/300 = 2V1/T2
T2 = 300 x 2 = 600K
Hence, T2 = 600 - 273 = 327