Answer: 1) Endothermic
2) Yes, absorbed.
3) 166.86 kJ will be absorbed.
Explanation:
1) To determine if a reaction is endothermic (heat is absorbed by the system) or exothermic (heat is released by the system), first calculate its change in Enthalpy, which is given by:
ΔH =
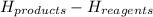
For the reaction 2Fe₂O₃(s) ⇒ 4FeO(s) + O₂(g):
Enthalpy of Reagent (Fe₂O₃(s))
Enthalpy of formation for Fe₂O₃(s) is - 822.2 kJ/mol
The reaction needs 2 mols of the molecule, so:
H = 2(-822.2)
H = - 1644.4
Enthalpy of Products (4FeO(s) + O₂(g))
Enthalpy of formation of O₂ is 0, because it is in its standard state.
Enthalpy of formation of FeO is - 272.04 kJ/mol
The reaction produces 4 mols of iron oxide, so:
H = 4(-272.04)
H = -1088.16
Change in Enthalpy:
ΔH =
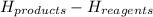
ΔH = - 1088.16 - (-1644.4)
ΔH = + 556.2 kJ/mol
The change in enthalpy is positive, which means that the reaction is absorving heat. Then, the chemical reaction is Endothermic.
2) When Fe₂O₃(s) reacts, heat is absorbed because it is an endothermic reaction.
3) Calculate how many mols there is in 94.2 g of Fe₂O₃(s):
n =

n =
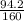
n = 0.6 mols
In the reaction, for 2 mols of Fe₂O₃(s), 556.2 kJ are absorbed. Then:
2 mols --------------- 556.2 kJ
0.6 mols ------------- x
x =
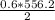
x = 167 kJ
It will be absorbed 167 kJ of energy, when 94.2 g of Fe₂O₃(s) reacts.