Answer: The equilibrium constant, K, would be greater than 1 at temperatures above 7269 Kelvin.
Explanation:-
Using Gibbs Helmholtz equation:
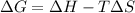
Where: T= Temperature =
 = enthalpy change = 181 kJ/mol = 181000 J/mol (1kJ=1000J)
= enthalpy change = 181 kJ/mol = 181000 J/mol (1kJ=1000J)
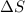 = entropy change= 24.9 J/Kmol
= entropy change= 24.9 J/Kmol
The Gibbs free energy is related to equilibrium constant by following relation:
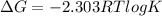
K > 1, when
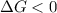
Putting the values in the equation:
For
 < 0,
< 0,
 >
>

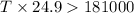
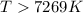
Thus the equilibrium constant, K, would be greater than 1 at temperatures above 7269 Kelvin.