Answer:
If 0.75 grams of iron (Fe) react, 0.85 grams of copper (Cu) will be produced.
Step-by-step explanation:
You know the following balanced reaction:
Fe + CuSO₄ ⇒ Cu + FeSO₄
By stoichiometry of the reaction (that is, the relationship between the amount of reagents and products in a chemical reaction), the following quantities react and are produced:
- Fe: 1 mole
- CuSO₄: 1 mole
- Cu: 1 mole
- FeSO₄: 1 mole
Being:
- Fe: 55.85 g/mole
- Cu: 63.54 g/mole
- S: 32 g/mole
- O: 16 g/mole
the molar mass of the compounds participating in the reaction is:
- Fe: 55.85 g/mole
- CuSO₄: 63.54 g/mole + 32 g/mole+ 4* 16 g/mole= 159.54 g/mole
- Cu: 63.54 g/mole
- FeSO₄: 55.85 g/mole + 32 g/mole+ 4* 16 g/mole= 151.85 g/mole
Then, by stoichiometry of the reaction, the amounts of reagent and product that participate in the reaction are:
- Fe: 1 mole*55.85 g/mole= 55.85 g
- CuSO₄: 1 mole* 159.54 g/mole= 159.54 g
- Cu: 1mole* 63.54 g/mole= 63.54 g
- FeSO₄: 1 mole* 151.85 g/mole= 151.85 g
Then you can apply a rule of three as follows: if 55.85 grams of Fe produces 63.54 grams of Cu, 0.75 grams of Fe how much mass of Cu does it produce?
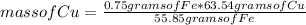
mass of Cu= 0.85 grams
If 0.75 grams of iron (Fe) react, 0.85 grams of copper (Cu) will be produced.