Answer:
T2 = 133.333°K
Step-by-step explanation:
Using Combined Gas Laws:
(600 torr)(10L)/500°K = (200 torr)(8L)/x°K
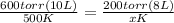
Cross multiply:
x°K (600 torr)(10L) = 500°K(200 torr)(8L)
Divide:
x°K = (500°K(200 torr)(8L))/(600 torr)(10L)
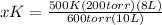
x = 400/3°K or 133.333°K