Answer:
The reaction would shift toward the reactants
When the reaction reach equilibrium the partial pressure of NH3 will be greater than 1atm
Step-by-step explanation:
For the reaction:
2NH₃(g) ⇄ N₂(g) + 3H₂(g)
Where K is defined as:
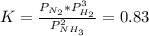
As initial pressures of all 3 gases is 1.0atm, reaction quotient, Q, is:
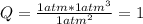
As Q > K, the reaction will produce more NH₃ until Q = K consuming N₂ and H₂.
Thus, there are true:
The reaction would shift toward the reactants
When the reaction reach equilibrium the partial pressure of NH3 will be greater than 1atm