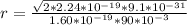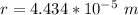Answer:
The radius is
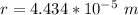
Step-by-step explanation:
From the question we are told that
The magnetic field is
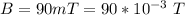
The electron kinetic energy is
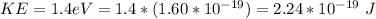
Generally for the collision to occur the centripetal force of the electron in it orbit is equal to the magnetic force applied
This is mathematically represented as
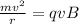
=>
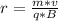
Where m is the mass of electron with values
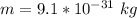
v is the escape velocity which is mathematically represented as
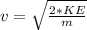
So
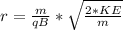
apply indices
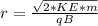
substituting values