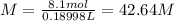Answer:
42.64 M
Step-by-step explanation:
Step 1: Given data
- Moles of HCl (solute): 8.1 mol
- Volume of the solution: 189.98 mL
Step 2: Convert the volume of the solution to liters
We will use the relationship 1 L = 1,000 mL.
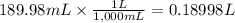
Step 3: Calculate the molarity of the solution
The molarity is equal to the moles of solute divided by the liters of solution.