Answer: Thus 234 kJ of energy are required to produce 1.00 kilogram of iron metal
Step-by-step explanation:
To calculate the number of moles , we use the equation:
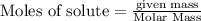
Putting values , we get:
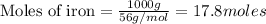 (1.00kg=1000g)
(1.00kg=1000g)
The balanced chemical reaction is:
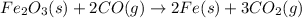
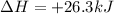
Given :
Energy released when 2 moles of
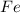 is produced = 26.3 kJ
is produced = 26.3 kJ
Thus Energy released when 17.8 moles of
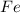 is produced =
is produced =
=
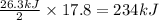
Thus 234 kJ of energy are required to produce 1.00 kilogram of iron metal