Answer:
4.95
Step-by-step explanation:
1.00 M H2A
1.38 m NaOH
Titration = 200.0 mL
Calculate moles of NaOH
=
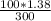 = 0.46
= 0.46
calculate moles of H2A
=
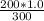 = 0.667
= 0.667
therefore the moles of acid left = moles of H2A - moles of NaOH
= 0.667 - 0.46 = 0.207
pka = - log( ka )
= - log ( 2.5 * 10^-5 ) = 4.61
calculate PH after 100 ml of 1.38 M NaOH have been added
PH = pka + log
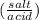
= 4.61 + log
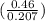 = 4.95
= 4.95