Answer:
Approximately
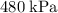 , assuming that this gas is an ideal gas.
, assuming that this gas is an ideal gas.
Step-by-step explanation:
- Let
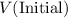 and
and
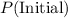 denote the volume and pressure of this gas before the compression.
denote the volume and pressure of this gas before the compression. - Let
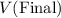 and
and
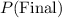 denote the volume and pressure of this gas after the compression.
denote the volume and pressure of this gas after the compression.
By Boyle's Law, the pressure of a sealed ideal gas at constant temperature will be inversely proportional to its volume. Assume that this gas is ideal. By this ideal gas law:
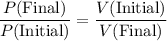 .
.
Note that in Boyle's Law,
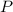 is inversely proportional to
is inversely proportional to
 . Therefore, on the two sides of this equation, "final" and "initial" are on different sides of the fraction bar.
. Therefore, on the two sides of this equation, "final" and "initial" are on different sides of the fraction bar.
For this particular question:
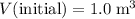 .
.
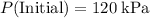 .
.
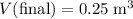 .
.- The pressure after compression,
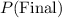 , needs to be found.
, needs to be found.
Rearrange the equation to obtain:
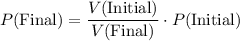 .
.
Before doing any calculation, think whether the pressure of this gas will go up or down. Since the gas is compressed, collisions between its particles and the container will become more frequent. Hence, the pressure of this gas should increase.
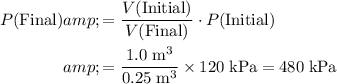 .
.