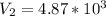Answer:
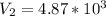
Step-by-step explanation:
This question is an illustration of ideal Gas Law;
The given parameters are as follows;
Initial Temperature = 25C
Initial Volume = 4.5 * 10³L
Required
Calculate the volume when temperature is 50C
NB: Pressure remains constant;
Ideal Gas Law states that;
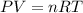
The question states that the pressure is constant; this implies that the constant in the above formula are P, R and n
Divide both sides by PT
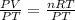
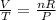
Represent
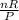 with k
with k
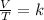
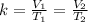
At this point, we can solve for the required parameter using the following;
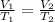
Where V1 and V2 represent the initial & final volume and T1 and T2 represent the initial and final temperature;
From the given parameters;
V1 = 4.5 * 10³L
T1 = 25C
T2 = 50C
Convert temperatures to degree kelvin
V1 = 4.5 * 10³L
T1 = 25 +273 = 298K
T2 = 50 + 273 = 323K
Substitute values for V1, T1 and T2 in
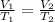
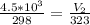
Multiply both sides by 323
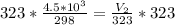
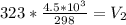
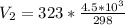
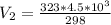
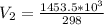
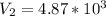
Hence, the final volume at 50C is