Answer:
4.08 grams
Step-by-step explanation:
Essentially, we're looking for the mass of HCl that "matches" 3.26 grams of magnesium hydroxide.
First, convert 3.26 grams of
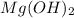 into moles by dividing by the molar mass. The molar mass of
into moles by dividing by the molar mass. The molar mass of
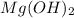 is 24.3 + 16 * 2 + 1 * 2 = 58.3 g/mol. So, 3.26 grams is equal to:
is 24.3 + 16 * 2 + 1 * 2 = 58.3 g/mol. So, 3.26 grams is equal to:
3.26 g ÷ 58.3 g/mol = 0.0559 mol
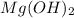
Notice that from the chemical equation, magnesium hydroxide and hydrochloric acid (HCl) have a ratio of 1 to 2. In other words, for every 0.0559 moles of
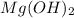 , there are 0.0559 * 2 = 0.112 moles of HCl.
, there are 0.0559 * 2 = 0.112 moles of HCl.
Finally, convert moles of HCl to grams by multiplying 0.112 by the molar mass, which is 1 + 35.45 = 36.45 g/mol:
0.112 mol HCl * 36.45 g/mol = 4.08 g HCl
The answer is thus 4.08 grams.
~ an aesthetics lover