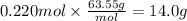Answer:
14.0 g
Step-by-step explanation:
Step 1: Write the balanced equation
2 Cu₂O + Cu₂S ⇒ 6 Cu + SO₂
Step 2: Calculate the moles corresponding to 10.5 g of copper (I) oxide
The molar mass of Cu₂O is 143.09 g/mol.
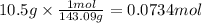
Step 3: Calculate the moles of copper produced from 0.0734 moles of copper (I) oxide
The molar ratio of Cu₂O to Cu is 2:6. The moles of Cu produced are 6/2 × 0.0734 mol = 0.220 mol
Step 4: Calculate the mass corresponding to 0.220 mol of copper
The molar mass of Cu is 63.55 g/mol.