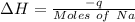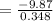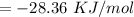Answer:
The correct answer to the following question will be Option A (-28.36 KJ). The further explanation is given below.
Step-by-step explanation:
The given values are:
Sodium's mass = 8 grams
Water's volume = 227 cm³
Molar mass = 23 g/mol
Density of water = 1 g/cm³
Specific heat capacity (
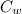 ) = 4.18 J/Kg
) = 4.18 J/Kg
As we know,
⇒
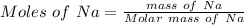
On putting the estimated values, we get
⇒
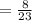
⇒
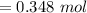
Now, Water's mass will be:
⇒
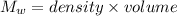

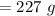
Change in temperature will be:
⇒
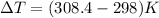
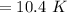
Heat released will be:
⇒
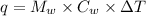
On substituting the estimated values, we get
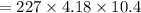
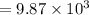
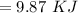
So that the change in the solution of Na will be:
⇒