Answer: The percent yield is, 93.4%
Step-by-step explanation:
First we have to calculate the moles of Na.
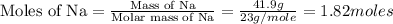
Now we have to calculate the moles of
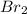
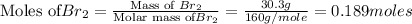
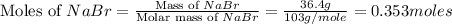
The balanced chemical reaction is,
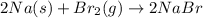
As, 1 mole of bromine react with = 2 moles of Sodium
So, 0.189 moles of bromine react with =
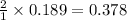 moles of Sodium
moles of Sodium
Thus bromine is the limiting reagent as it limits the formation of product and Na is the excess reagent.
As, 1 mole of bromine give = 2 moles of Sodium bromide
So, 0.189 moles of bromine give =
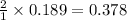 moles of Sodium bromide
moles of Sodium bromide
Now we have to calculate the percent yield of reaction
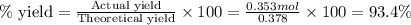
Therefore, the percent yield is, 93.4%