Answer: The pH of the solution is 11.2
Step-by-step explanation:
Molarity of a solution is defined as the number of moles of solute dissolved per liter of the solution.
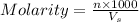
where,
n = moles of solute
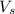 = volume of solution in ml
= volume of solution in ml
moles of
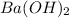 =
=
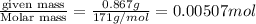 (1g=1000mg)
(1g=1000mg)
Now put all the given values in the formula of molality, we get
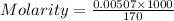
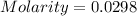
pH or pOH is the measure of acidity or alkalinity of a solution.
pH is calculated by taking negative logarithm of hydrogen ion concentration.
![pOH=-\log [OH^-]](https://img.qammunity.org/2021/formulas/chemistry/college/hdm1ob4dj6mx2sy3kobrrj91lzbh3927bk.png)
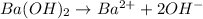
According to stoichiometry,
1 mole of
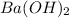 gives 2 mole of
gives 2 mole of
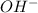
Thus 0.0298 moles of
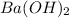 gives =
gives =
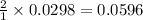 moles of
moles of
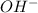
Putting in the values:
![pOH=-\log[0.0596]=2.82](https://img.qammunity.org/2021/formulas/chemistry/college/ggiucvw9ktmcq558biv5tfayke8hosferw.png)
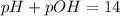
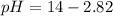
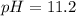
Thus the pH of the solution is 11.2