Answer: The molarity of the borax solution is 0.107 M
Step-by-step explanation:
The neutralization reaction is:
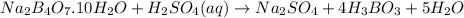
According to neutralization law:
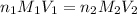
where,
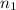 = basicity of
= basicity of
 = 2
= 2
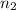 = acidity of borax = 2
= acidity of borax = 2
 = concentration of
= concentration of
 = 1.03 M
= 1.03 M
 = concentration of borax =?
= concentration of borax =?
 = volume of
= volume of
 = 2.07ml
= 2.07ml
 = volume of borax = 20.0 ml
= volume of borax = 20.0 ml
Now put all the given values in the above law, we get the molarity of borax:
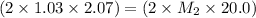
By solving the terms, we get :
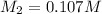
Thus the molarity of the borax solution is 0.107 M