Answer:
514 cal
Step-by-step explanation:
In order to calculate the lost heat by the amount of water you first take into account the following formula:
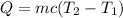 (1)
(1)
Q: heat lost by the amount of water = ?
m: mass of the water
c: specific heat of water = 1cal/g°C
T2: final temperature of water = 11°C
T1: initial temperature = 12°C
The amount of water is calculated by using the information about the density of water (1g/ml):
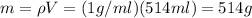
Then, you replace the values of all parameters in the equation (1):
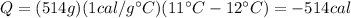
The amount of water losses a heat of 514 cal