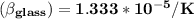Answer:
the coefficient of volume expansion of the glass is
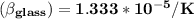
Step-by-step explanation:
Given that:
Initial volume of the glass flask = 1000 cm³ = 10⁻³ m³
temperature of the glass flask and mercury= 1.00° C
After heat is applied ; the final temperature = 52.00° C
Temperature change ΔT = 52.00° C - 1.00° C = 51.00° C
Volume of the mercury overflow = 8.50 cm^3 = 8.50 × 10⁻⁶ m³
the coefficient of volume expansion of mercury is 1.80 × 10⁻⁴ / K
The increase in the volume of the mercury = 10⁻³ m³ × 51.00 × 1.80 × 10⁻⁴
The increase in the volume of the mercury =
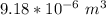
Increase in volume of the glass = 10⁻³ × 51.00 ×
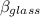
Now; the mercury overflow = Increase in volume of the mercury - increase in the volume of the flask
the mercury overflow =
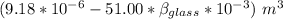
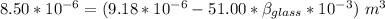
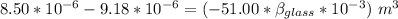
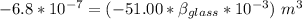
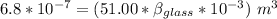
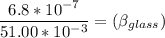
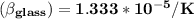
Thus; the coefficient of volume expansion of the glass is