Answer:
See the explanation
Step-by-step explanation:
In this case, we have to keep in mind that in the monosubstituted product we only have to replace 1 hydrogen with another group. In this case, we are going to use the methyl group
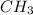 .
.
In the axial position, we have a more steric hindrance because we have two hydrogens near to the
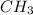 group. If we have more steric hindrance the molecule would be more unstable. In the equatorial positions, we don't any interactions because the
group. If we have more steric hindrance the molecule would be more unstable. In the equatorial positions, we don't any interactions because the
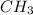 group is pointing out. If we don't have any steric hindrance the molecule will be more stable, that's why the molecule will the equatorial position.
group is pointing out. If we don't have any steric hindrance the molecule will be more stable, that's why the molecule will the equatorial position.
See figure 1
I hope it helps!