Answer:
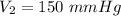
Step-by-step explanation:
Given
Initial Pressure = 300 mmHg
Initial Volume = 380 L
Required
Volume of the gas at standard pressure
This can be solved using Boyle's law;
Which states that
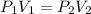
Where
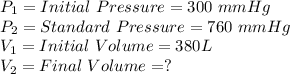
Substitute the above values in the Boyle's law equation
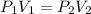 becomes
becomes
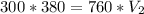
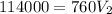
Divide both sided by 760
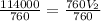
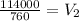
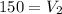
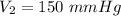
Hence, the volume of the gas as standard pressure is 150 mmHg