Answer:
53.8 L
Explanation:
We can use the ideal gas formula that relates pressure, volume and temperature:
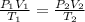
where P1 = initial pressure = 1.0 atm
V1 = initial volume = 45 L
T1 = initial temperature = 25°C = 25 + 273 = 298 K
P2 = final pressure = 0.78 atm
V2 = final volume
T2 = final temperature = 5.0°C = 5 + 273 = 278 K
Therefore, the final volume will be:
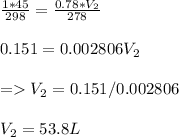
Its volume is 53.8 L