Answer:
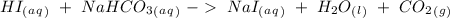
Step-by-step explanation:
In this case, we will have a neutralization reaction. We have a base (
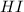 ) and a base (
) and a base (
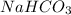 ). Additionally, we have a strong acid and a strong base, therefore both will be soluble on water, so we will have an aqueous state for these compounds. If we will have a neutralization reaction, we will have as a salt as a product. With this in mind the reaction would be:
). Additionally, we have a strong acid and a strong base, therefore both will be soluble on water, so we will have an aqueous state for these compounds. If we will have a neutralization reaction, we will have as a salt as a product. With this in mind the reaction would be:
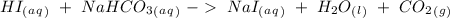
All the sodium salts are soluble in water, therefore we will have an aqueous state. Water is a liquid and carbon dioxide is a gas.
I hope it helps!