Given Information:
Inlet velocity = Vin = 25 m/s
Exit velocity = Vout = 250 m/s
Exit Temperature = Tout = 300K
Exit Pressure = Pout = 100 kPa
Required Information:
Inlet Temperature of argon = ?
Inlet Temperature of helium = ?
Inlet Temperature of nitrogen = ?
Answer:
Inlet Temperature of argon = 360K
Inlet Temperature of helium = 306K
Inlet Temperature of nitrogen = 330K
Step-by-step explanation:
Recall that the energy equation is given by
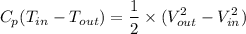
Where Cp is the specific heat constant of the gas.
Re-arranging the equation for inlet temperature
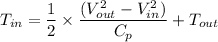
For Argon Gas:
The specific heat constant of argon is given by (from ideal gas properties table)
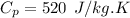
So, the inlet temperature of argon is
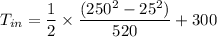
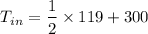
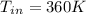
For Helium Gas:
The specific heat constant of helium is given by (from ideal gas properties table)
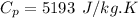
So, the inlet temperature of helium is
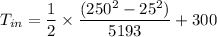
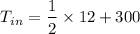
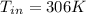
For Nitrogen Gas:
The specific heat constant of nitrogen is given by (from ideal gas properties table)
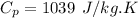
So, the inlet temperature of nitrogen is
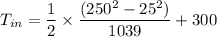
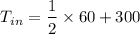
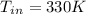
Note: Answers are rounded to the nearest whole numbers.