Answer:
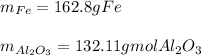
Step-by-step explanation:
Hello,
In this case, by the following balanced reaction:
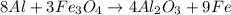
The first step is to identify the limiting reactant, for which we compute the available moles of aluminium in 225.0 g by using its atomic mass:
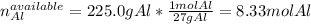
Next. we compute the consumed moles of aluminium by 225.0 g of iron (II,III) oxide by using its molar mass and the 8:3 molar ratio between them:
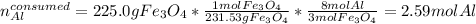
In such a way, since more Al is available, we conclude it is in excess and iron (II,III) oxide is the limiting reactant, therefore, we can compute the produced grams of both iron and aluminium oxide as shown below:
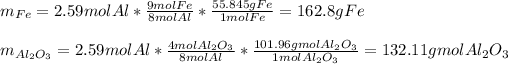
Best regards.