Answer:
2.29 g of N2
Step-by-step explanation:
We have to start with the chemical reaction:
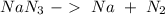
The next step is to balance the reaction:
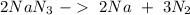
We can continue with the mol calculation using the molar mass of
 (65 g/mol), so:
(65 g/mol), so:
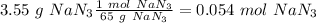
Now, with the molar ratio between
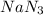 and
and
 we can calculate the moles of
we can calculate the moles of
 (2:3), so:
(2:3), so:
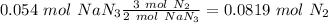
With the molar mass of
 we can calculate the grams:
we can calculate the grams:
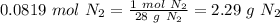
I hope it helps!