Answer:
See the explanation below. Thanks!
Step-by-step explanation:
The structure of an amino acid allows it to act as both an acid and a base. An amino acid has this ability because at a certain
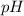 value (different for each amino acid) nearly all the amino acid molecules exist as zwitter-ions. If acid is added to a solution containing the zwitter-ion, the carboxylate group captures a hydrogen (
value (different for each amino acid) nearly all the amino acid molecules exist as zwitter-ions. If acid is added to a solution containing the zwitter-ion, the carboxylate group captures a hydrogen (
 ) ion, and the amino acid becomes positively charged. If base is added, ion removal of the
) ion, and the amino acid becomes positively charged. If base is added, ion removal of the
 ion from the amino group of the zwitter-ion produces a negatively charged amino acid. In both circumstances, the amino acid acts to maintain the pH of the system—that is, to remove the added acid
ion from the amino group of the zwitter-ion produces a negatively charged amino acid. In both circumstances, the amino acid acts to maintain the pH of the system—that is, to remove the added acid
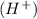 or base
or base
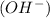 from solution.
from solution.