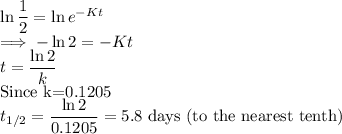Complete Question
Situation: A 25 gram sample of a substance used for drug research has a k-value of 0.1205.
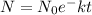
 = initial mass(at time t=0)
= initial mass(at time t=0) - N= mass at a time t
- K= a positive constant that depends on the substance itself and on the units used to measure time
- T=time, in days
Find the substance's half-life in days, round to the nearest tenth.
Answer:
5.8 days
Explanation:
The decay model for drugs and radioactive substances is given as
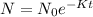
The half-life of any substance is the time it takes for the substance to decay to half its initial amount. That is the period it takes for:
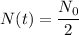
If we substitute this into the model, we obtain:
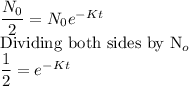
We can solve for t.
Taking the natural logarithm of both sides