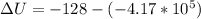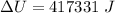Answer:
The change in internal energy is
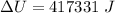
Step-by-step explanation:
From the question we are told that
The initial volume is
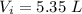
The final volume is
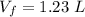
The value of the external pressure is

The energy released by the gas is
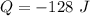
The negative sign show that energy is released from the system
Generally the workdone on the gas is mathematically represented as
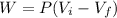
substituting values
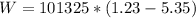
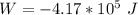
The change in internal energy is mathematically evaluated as
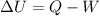
substituting values