Answer:
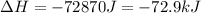
Step-by-step explanation:
Hello,
In this case, for the computation of the total energy, we must consider two processes:
1. Condensation of steam (heat of condensation is the negative of heat of vaporization).
2. Cooling of hot water (we use the specific heat of water).
Thus, we write:
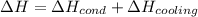
For each term, we have:
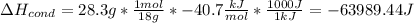
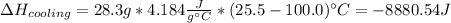
Therefore, the total energy results:
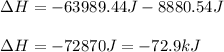
Regards.