Answer:
See explanation
Step-by-step explanation:
In this case, we have 2 types of reactions.
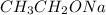 is a strong base but only has 2 carbons therefore we will have less steric hindrance in this base. So, the base can remove hydrogens that are bonded on carbons 1 or 6, therefore, we will have a more substituted alkene (1-methylcyclohex-1-ene).
is a strong base but only has 2 carbons therefore we will have less steric hindrance in this base. So, the base can remove hydrogens that are bonded on carbons 1 or 6, therefore, we will have a more substituted alkene (1-methylcyclohex-1-ene).
For the
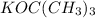 we have more steric hindrance. So, we can remove only the hydrogens from carbon 7 and we will produce a less substituted alkene (methylenecyclohexane).
we have more steric hindrance. So, we can remove only the hydrogens from carbon 7 and we will produce a less substituted alkene (methylenecyclohexane).
See figure 1
I hope it helps!