Answer:
The correct option is;
B) 179 g
Step-by-step explanation:
The parameters given are;
Mass of H₂ that takes part in the reaction = 2.23 g
Molar mass of hydrogen gas, H₂ = 2.016 g
Number of moles, n, of hydrogen gas H₂ is given by the relation;
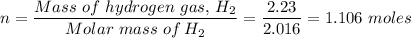
Chemical equation for the reaction;
H₂ + Br₂ → 2HBr
Given that one mole of H₂ reacts with one mole of Br₂ to produce two moles of HBr
1.106 mole of H₂ will react with 1.106 mole of Br₂ to produce 2 × 1.106 which is 2.212 moles of HBr
The molar mass, of HBr = 80.91 g/mol
The mass of HBr produced = Molar mass of HBr × Number of moles of HBr
The mass of HBr produced = 80.91 × 2.212 = 178.997 g ≈ 179 grams
Therefore, the correct option is B) 179 g.