Answer:
The mass of water that uses 7500 J to increase the temperature from 2°C to 3°C is 179.543 grams
Step-by-step explanation:
The given parameters are
Change in Heat , ΔH = 7500 J
The change in temperature ΔT = 2°C to 3°C = 3 - 1 = 1°C
The specific heat capacity for water is given as Δc = 4.184 J/(g·°C)
The heat of fusion,
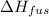 = 334 J/g
= 334 J/g
The heat of vaporization,
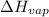 = 2260 J/g
= 2260 J/g
The formula for heat capacity is given as follows;
ΔH = m × Δc × ΔT
Given that the temperature of the process is above the melting point temperature of 0°C and below the boiling point temperature of 100°C, we make use only of the heat capacity of liquid water
Therefore, we have;
7500 J = m × 4.184 × 1
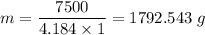
The mass of water that uses 7500 J to increase the temperature from 2°C to 3°C = 179.543 grams.