Answer: The minimum
![[Ag^(+)]](https://img.qammunity.org/2021/formulas/chemistry/college/olsa8shvj9cd8zwjhfv1es86wljsrbwm1g.png) concentrations required to precipitate out the anions is
concentrations required to precipitate out the anions is
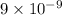 M.
M.
Step-by-step explanation:
We know that,
 for AgCl is
for AgCl is
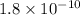
and,
 for
for
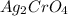 is
is
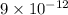
Now, we will calculate the concentration of at which these ions precipitate out are as follows.
For AgCl :
![[Ag^(+)] = (K_(sp))/([Cl^(-)])](https://img.qammunity.org/2021/formulas/chemistry/college/z4uqdcmimy4kjes7b43xneadgr00esqr20.png)
=
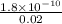
=
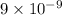 M
M
For
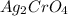 :
:
![[Ag^(+)]^(2) = (K_(sp))/(CrO^(2-)_(4))](https://img.qammunity.org/2021/formulas/chemistry/college/r9wmfh5a0mptgeouhqjwunezoruhpdgaxf.png)
=
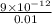
=
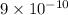
![[Ag^(+)] = \sqrt{(9 * 10^(-9))}](https://img.qammunity.org/2021/formulas/chemistry/college/w4bbak7x8bz84fijn9lel07cyxbs2d09ql.png)
=
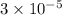 M
M
This shows that concentration of ions in AgCl is less than the concentration of AgCl will precipitate first.