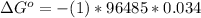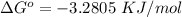Complete Question
The complete question is shown on the first uploaded image
Answer:
The change in reduction potential is
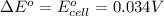
The change in standard free energy is
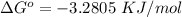
Step-by-step explanation:
From the question we are told that
At the anode
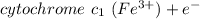 ⇔
⇔
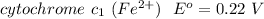
At the cathode
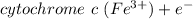 ⇔
⇔
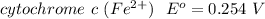
The difference in the reduction potential is mathematically represented as
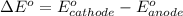
substituting values
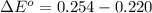
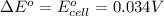
The change in the standard free energy is mathematically represented as
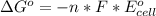
Where F is the Faraday constant with value F = 96485 C
and n i the number of the number of electron = 1
So