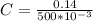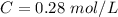Complete Question
A chemist prepares a solution of barium chlorate BaClO32 by measuring out 42.g of barium chlorate into a 500.mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in /molL of the chemist's barium chlorate solution. Be sure your answer has the correct number of significant digits.
Answer:
The concentration is
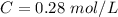
Step-by-step explanation:
From the question we told that
The mass of
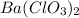 is
is
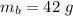
The volume of the solution
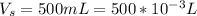
Now the number f moles of
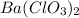 in the solution is mathematically represented as
in the solution is mathematically represented as
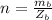
Where
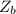 is the molar mass of
is the molar mass of
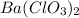 which a constant with a value
which a constant with a value
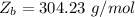
Thus
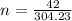
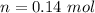
The concentration of
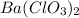 in the solution is mathematically evaluated as
in the solution is mathematically evaluated as
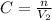
substituting values