Answer:
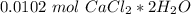
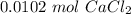
Step-by-step explanation:
For this question, we have to start with the molar mass calculation of
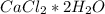 . For this, we have to know the atomic mass of each atom:
. For this, we have to know the atomic mass of each atom:
O: 16 g/mol
Cl: 35.45 g/mol
H: 1 g/mol
Ca: 40 g/mol
If we take into account the amount of each atom in the formula we will have:
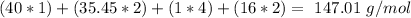
So, in 1 mol of
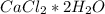 we will have 147.01 g. Now we can do the conversion:
we will have 147.01 g. Now we can do the conversion:
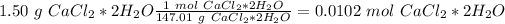
Additionally, in 1 mol of
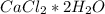 we will have 1 mol of
we will have 1 mol of
 . Therefore, we have a 1:1 mol ratio . With this in mind, we will have the same number of moles for
. Therefore, we have a 1:1 mol ratio . With this in mind, we will have the same number of moles for

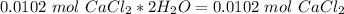
I hope it helps!