Answer: The final concentration is 0.16 M.
Step-by-step explanation:
According to the dilution law:
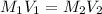
where,
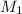 = molarity of stock solution = 1.60 M
= molarity of stock solution = 1.60 M
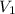 = volume of stock solution = 54.0 ml
= volume of stock solution = 54.0 ml
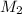 = molarity of diluted solution = ?
= molarity of diluted solution = ?
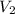 = volume of diluted solution = 218 ml
= volume of diluted solution = 218 ml
Putting these values:
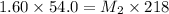
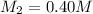
Now 109 ml of this diluted solution is further diluted by adding 161 mL of water.
Again applying dilution law:
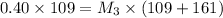
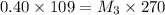
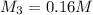
Thus the final concentration is 0.16 M