Answer:
N' = 1.17*10^21 molecules/m^3
Step-by-step explanation:
In order to calculate the surface density in Titan's atmosphere, in molecules per cubic meter, you use the following formula for ideal gases:
 (1)
(1)
P: pressure = 1.5 Earth-atmospheres = 1.5 atm
R: ideal gas constant = 8.205 m^3.atm.mol^-1.K^-1
T: temperature in Titan = 94K
n: number of moles
To obtain the number of moles per cubic meter you write the equation (1) as follow:
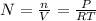 (2)
(2)
N: moles per cubic meter
You use the Avogadro's number for the number of molecules:
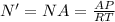 (3)
(3)
A: Avogadro's number = 6.022*109^23 molecules/mol
N': molecules per cubic meter
You replace the values of all parameters in the equation (3):
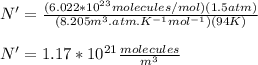
The surface density of molecules in Titan is 1.17*10^21 molecules/m^3