Answer:
E = 0.965eV
Step-by-step explanation:
In order to calculate the minimum energy needed to eject the electrons you use the following formula:
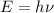 (1)
(1)
h: Planck' constant = 6.626*10^{-34}J.s
v: threshold frequency = 2.47*10^14 s^-1
You replace the values of v and h in the equation (1):
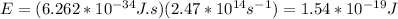
In electron volts you obtain:
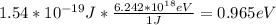
The minimum energy needed is 0.965eV