Answer:
The barrier has to be 34.23 kJ/mol lower when the sucrose is in the active site of the enzyme
Step-by-step explanation:
From the given information:
The activation barrier for the hydrolysis of sucrose into glucose and fructose is 108 kJ/mol.
In this same concentration for the glucose and fructose; the reaction rate can be calculated by the rate factor which can be illustrated from the Arrhenius equation;
Rate factor in the absence of catalyst:
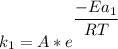
Rate factor in the presence of catalyst:
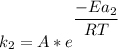
Assuming the catalyzed reaction and the uncatalyzed reaction are taking place at the same temperature :
Then;
the ratio of the rate factors can be expressed as:
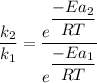
![(k_2)/(k_1)={ \frac {e^([ Ea_1 - Ea_2 ] )}{RT} }}](https://img.qammunity.org/2021/formulas/chemistry/college/11kiswtlyc3x3bytmvvk4z5q5kgc81n08a.png)
Thus;
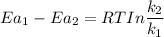
Let say the assumed temperature = 25° C
= (25+ 273)K
= 298 K
Then ;
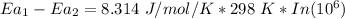
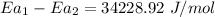
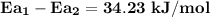
The barrier has to be 34.23 kJ/mol lower when the sucrose is in the active site of the enzyme