Answer:
See explanation
Step-by-step explanation:
We have to remember that for any equilibrium expression constant we can use the general reaction:
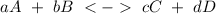
With a equilibrium expression:
![Keq~=~([C]^c[D]^d)/([A]^a[B]^b)](https://img.qammunity.org/2021/formulas/chemistry/college/c4rpa6288k1oss84ajmtfdkrj7g5zqax2i.png)
We have to divide products by reagents and use the coefficient (in the balanced reaction) as exponents. So:
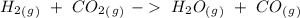
The reaction is already balanced. So, we can write the equilibrium expression:
![Keq~=~([H_2O][CO])/([H_2][CO_2])](https://img.qammunity.org/2021/formulas/chemistry/college/5eiji4ahup6fmfz21sm5k5k2bghous9k0v.png)
In this case, all the compounds have gaseous state (g). So, we can change the concentration ([]) by the pressure of each compound:
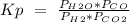
I hope it helps!