Answer:
The original volume of the first bar is half of the original volume of the second bar.
Step-by-step explanation:
The coefficient of cubic expansivity of substances is given by;
γ = ΔV ÷ (
 Δθ)
Δθ)
Given: two metal bars with equal change in volume, equal change in temperature.
Let the volume of the first metal bar be represented by
 , and that of the second by
, and that of the second by
 .
.
Since they have equal change in volume,
Δ
 = Δ
= Δ
 = ΔV
= ΔV
For the first metal bar,
2γ = ΔV ÷ (
 Δθ)
Δθ)
⇒ Δθ = ΔV ÷ (2γ
 )
)
For the second metal bar,
γ = ΔV ÷ (
 Δθ)
Δθ)
⇒ Δθ = ΔV ÷ (
 γ)
γ)
Since they have equal change in temperature,
Δθ of first bar = Δθ of the second bar
ΔV ÷ (2γ
 ) = ΔV ÷ (
) = ΔV ÷ (
 γ)
γ)
So that;
(1 ÷ 2
 ) = (1 ÷
) = (1 ÷
 )
)
2
 =
=

 =
=
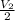
Thus, original volume of the first bar is half of the original volume of the second bar.